A New Approach To Plant-Derived Mordants
Aluminum metal salts have been in demand by natural dyers for millennia because they are highly effective at strongly bonding natural fibres and natural dyes to each other, through what is known in chemistry as a molecular ‘coordination complex’, and because, unlike most other mordants and mordant assists, they generally do not affect the final dye colour, other than brightening it. So, when it comes to final colour, they let the natural dye itself ‘do the talking’.
In the past, alum was mined from aluminum-rich soils/sediments, and put through elaborate processing to extract and purify the mordant component. Much the same extraction and purification happens to this day.
In addition, as many natural dyers know, there are some plant species that are well-known hyper-accumulators of aluminum (the third most plentiful element, and the most plentiful metal, on Earth), drawing aluminum up from the soil through their roots. Some of the better known examples include Lycopodium spp. (Clubmosses) that were historically used in Europe, as well as various members of the Asteraceae, Fabacaea, Brassicaceae, and Symplocaceae plant families.
Lycopodium species growing among Maianthemum canadense at Mamie’s Schoolhouse. Once widespread in the northern hemisphere, many Lycopodium species are now extinct or ecologically threatened, due to over-harvesting and deforestation. They rely on a stable forest ecosystem for survival, often growing in association with particular hardwood species. Never harvest without first confirming a secure ecological status with local environmental authorities.
What Are Metal Salts
SOURCE: Wikimedia Commons
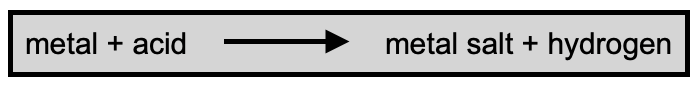
A metal salt forms when there is a reaction between a metal and an acid. Hydrogen is released in the process.
Some metal salts are soluble (will dissolve) in water, which is a handy property for natural dyers, since it means that, if we work with a soluble metal salt mordant, our mordant bath can penetrate into the interior of our fibres, ensuring thorough mordanting, and subsequent dye uptake.
Metal salts usually have two-part names. The first tells you what metal was involved in the reaction shown above, and the second tells you what acid was involved. Here are some common fibre mordant examples…
ferrous acetate = iron (Fe) + acetic acid
aluminum sulfate = aluminum + sulfuric acid
titanium oxalate = titanium + oxalic acid
Other Metal Salts
Of course, many other metal salts have been used historically as mordants (zinc, chrome, lead, cadmium, etc.). Since the advent of modern scientific research methods over the last few centuries, demonstrating the adverse human and ecological health impacts of some previously commonly used mordants and dye assists, responsible contemporary natural dyers now restrict themselves to the use of a small number of mordants that have minimal/no adverse impacts when used properly.
The Downside of Alum
While alum certainly has its charms - it’s easy to work with, is non-toxic, a little goes a long way, and it can produce beautiful, long-lasting dye results, I am very concerned about the ecological devastation being wrought across our beautiful, benighted planet. I believe strongly in re-localizing our economies and our lives as much as possible, to significantly reduce the ecosphere-destroying footprint of our modern way of life. This motivates me to investigate ways in which my life, and my natural dye practice, can learn to respectfully co-exist with my local environment, and to explore how to sustainably use resources from my own region as much as possible.
As a natural dye educator, and admin of the Natural Dye Education Facebook Group, I often have opportunity to educate newcomers to natural dyeing about the benign nature of alum, when used properly. Yet, the fact remains that while it is relatively benign at the user end, it is not at the production end. It is a mined product - that begins its journey to us from open pit mining operations that destroy wildlife habitat, pollute surrounding waterways, and strip the Earth of precious topsoil, from which bauxite is then extracted. Bauxite is then processed in industrial facilities - in energy and chemical intensive processes - to eliminate other soil components, leaving pure aluminum, which is then processed further to produce various aluminum salts for use in a range of industries, and by natural dyers. Half of all topsoil on our planet has been lost in the last, short 150 years. It is the means by which all life on our planet exists, so the idea of topsoil mining being required so that I can enjoy long-lasting colour on my fibres hasn’t sat well with me for some time.
Historic First Nations Practices
I have been researching the natural dye practices of pre-colonial and post-colonial Canada for many years. I knew from my reading that some of the First Nations of Canada (the pre-colonial peoples) used plants in their natural dye practices that did not seem to have a role in imparting colour, particularly the use of highly acidic berries whose colour comes from unstable anthocyanins (flavonoid molecules responsible for the black/blue/purple/red hues of many flowers and edible plants, and that degrade quickly when exposed to heat and/or light). So I have been curious about the role the highly acidic plants played in helping First Nations’ dyers achieve their desired dye result.
My working theory for some time has been that they observed that when they employed an acid in their bath, their dye results were richer and longer-lasting. They may have also observed how the use, or non-use, of an acid affected results from dye baths using pots of different materials. I suspect that their use of an acidic plant ingredient, that had little/no role in imparting colour, helped to extract metal salts - either from metal bioaccumulator species also in the pot and/or from the dye container itself. Historically, peoples around the world didn’t have our modern, coated cookware. Instead, they worked with reactive metal pots and/or earthenware containers which, made from various clays, would have contained metals naturally found in the clay soils used. Metals in soils are plentiful - aluminum being the most plentiful and iron being the second most plentiful metals on Earth. In addition, our natural dye ancestors often employed slower methods compared to many modern dyers, affording greater opportunity for maximum extraction of compounds helpful to achieving beautiful, long-lasting dye results.
Then, in about 2012, I came across a detailed 2009 academic research study of such historical practices, in which the authors came to the same conclusion, at least in regards to the use of acids to pull metal out of reactive dye pots ( Bohr, R., & Lindsay, A. (2009). “Dyeing Commodities whether in Roote or floure”: Reconstructing Aboriginal Dye Techniques from Documentary and Museum Sources. Material Culture Review, 69. Retrieved from https://journals.lib.unb.ca/index.php/MCR/article/view/18145).
I have seen a couple of other natural dyers acknowledge a historic tannin/acid dye method used by pre-colonial peoples of both North and South America, but I think they may be missing another potentially key component of the reaction(s) being created by these ingredients - the ability of an acid ingredient to react with a metal source - whether a plant, the dye container, or both - thus creating a metal salt within the bath, with fibre mordanting properties.
Native berries at Mamie’s Schoolhouse - on the left, the very acidic Prunus virginiana (Chokecherry) and on the right, Cornus canadensis (Bunchberry).
Other Plant Bioaccumulators Of Metals
The ability of some plants to intensively accumulate metals (and other substances) from soil has been known since at least the 16th century. Yet, for the most part, this knowledge seems to be predominantly employed only in environmental remediation efforts. Governments and industry routinely use plants for phytoremediation of former industrial sites, using specific plants to de-contaminate the soil.
The other area of endeavour where there seems to be wide acknowledgement of the ability of certain plants to transport desirable substances from the soil to the surface is in agriculture, particularly organic and permaculture agricultural models.
However, there are now some small-scale initiatives exploring the capacity for metal mining from plants as renewable resources, without the destructive ecological footprint of current industrial mining practices, reflecting a growing awareness of the potential of plants as an important source of metals for a range of applications.
left to right: native Vaccinium angustifolium (Lowbush Blueberry), a known bioaccumulator of copper and nickel - native salix spp. (willow), a known bioaccumulator of zinc and cadmium - and introduced Hydrangea macrophylla (hydrangea), a known bioaccumulator of aluminum. all growing at Mamie’s Schoolhouse.
Not all plants are metal bioaccumulators. A plant has to have evolved to have the right genetics to manage the processes for accessing, transporting, and utilizing soil metals. Also, soil conditions are significant. Acidic soil conditions, for the reasons noted above, convert soil metals into more soluble forms, that are then ‘bioavailable’ to plants. So, bioaccumulators tend to grow well in acidic soil conditions, and have the requisite genetics to convert soil metals into a useful part of the plant’s physiology (though, there are some plants that are very effective bioaccumulators, but they sequester, rather than use, the accumulated metals).
So, if we look at the the pink and white colour of the sepals in the image of my Hydrangea shrub above, it tells us that this shrub is not growing in acidic soil. This is because research evidence tells us that acidic soils produce blue and purple sepals on Hydrangea shrubs. Therefore, even though Hydrangea possesses the genetics to bioaccumulate aluminum, mine is currently missing the other key criteria needed for effective bioaccumulation. So no aluminum for me from my Hydrangea shrub! At least, not yet, as I intend to acidify the soil it’s growing in, so that I can test the aluminum bioaccumulation down the road (it will likely take several years for the necessary transformation).
Amongst the subset of plants that do bioaccumulate soil metals, there is variation in where they store it. Some retain the metal(s) in their roots, so that’s not particularly helpful to the natural dyer looking for a renewable source of plant mordants. But other species transport the metals to their aerial (above ground) parts, making them more accessible to us. The concentration of metal a bioaccumulater plant has in its aerial parts can be divided by the concentration in its roots to calculate what is known as the translocation factor (TF). A TF value of over 1 means that a plant is a good candidate for harvesting metals from its above ground parts.
So, as I started to more intensively look into the potential of easily available plants, that I could grow or responsibly forage, as potential sources of metal salt mordants, the first challenge was that there seemed to be so little comprehensive information available on plant bioaccumulators. But I did eventually come across the Global Hyperaccumulator Database, a mining industry initiative, housed at Australia’s University of Queensland. One of the first things you will note, is that the vast majority of known hyperaccumulators are found only in the tropics or sub-tropics.
However, I have also found research studies on a range of other plants, not in the above database, including many in the temperate regions of the world, that have been shown to be effective bioaccumulators. It requires some sifting through open source online academic research portals and journals to find them, so that can be challenging for someone who doesn’t have an academic or public policy background, but the information is out there.
left to right: invasive plantago major (broadleaf plantain) and native vibernum trilobum (highbush cranberry) berries. both growing at Mamie’s Schoolhouse.
Solubility of Metals
In chemistry, there is something known as the Reactivity Series of Metals. It is a hierarchy of metal elements, that explains which are least or most likely to react with something else (the air, or a solvent such as water or an acid, etc.). Within the overall hierarchy is a subset of metals that are reactive to acids - by exposing them to an acid, they are likely to form a metal salt. Knowing this helps us to understand which metals we might have a chance of extracting from plant material, using an acid easily available to us.
Testing Local Plants As Sources Of Metal Salt Mordants
So, having done research on some of the key considerations, for one of my early trials, I decided to use the following.
Bioaccumulator: Plantago major (Broadleaf Plantain)
a known bioaccumulator of iron, cadmium, and lead (where there is lead in the soil, which there isn’t on my property) - using the Reactivity Series of Metals noted above, we know that iron is more reactive than cadmium, so is more likely to react with my acid to form a ferrous metal salt
globally wide-spread, and often considered a ‘weed’/invasive in many parts of the world (originally native to Europe and Asia)
grows extensively where I live
known to contain high quantities of gallic tannins, which tend to be light coloured, and this was borne out by a preliminary test I did by soaking some chopped leaves in hottest tap water overnight, which produced quite a clear extraction
used leaves at 400% WOF (weight of fibre)
Acid: Vibernum trilobum (Highbush Cranberry)
a very acidic native berry
pH started at 3.4, dropping to 2.9 with longer extraction
pH comparable to acids used industrially to extract metals from various substances, such as sulphuric acid (pH of about 2.8 ) and hydrochloric acid (pH of about 3)
used berries at 200% WOF
First, I simmered the berries to speed up the degradation of the anthocyanins (in order to reduce colour imparted to the cloth during the mordanting process), and also to extract the acids. Then I added the bioaccumulator leaves (chopped up) and simmered. Heat will darken tannins, and I believe that most of the resulting colour on the plant mordanted fibres (scroll below) is the result of the bioaccumulator tannins, not the berry anthocyanins.
Preliminary Results
I used wool, silk, linen, and organic cotton fabric samples.
I also did control samples, using more ‘traditional’ metal salt mordant powder methods, as follows: cotton and linen - oak gall tannin, then aluminum acetate; wool and silk - potassium aluminum sulphate.
The plant mordanted fibres were left to soak for 24 hours in the bioaccumulator/acid combination bath, rinsed and dried.
Top row, left to right: plant mordanted wool, silk, organic cotton, linen
Bottom row, left to right: control samples of wool, silk (the cotton and linen aren’t shown, but were as white as the silk)
I then chose to dye all samples with Coreopsis tinctoria (Dyer’s Coreopsis), Rubia tinctorum (Madder), and Reseda luteola (Weld).
In the images below, MS = metal salt (the control samples), P = plant mordanted.
You can see that something funky happened to my Reseda luteola protein fibres. The dye bath was closely monitored, and did not overheat, so I am currently assuming that there is something in the chemistry of the bioaccumulator and acid plant constituents in the mordanted fibres that did not agree with Weld’s chemistry. It’s one of the many question marks that I am still investigating. However, overall, I was pleased with the dye results Some are more, others less, like the colour achieved using the traditional metal salt mordants, but either way, most of the colours achieved are rich and attractive, and maybe are the beginning of a new lexicon of natural dye colour.
I have just completed a three month long lightfast test of all samples. I chose such a long period, as when I started, it was the middle of Winter, with shorter days and a weaker sun than at other times of the year.
I am very pleased with the results.
I’ll be posting images of the lightfast test results for the above, as well as the actual metal readings I took of my water source (baseline reading) and of my bioaccumulator/acid bath, in my next Blog post. You can subscribe above, if you want to be notified when a new Blog post is published.
I’m also currently working on the following tests, and will be publishing their results over a series of upcoming blog posts.
different bioaccumulator plants, different acid sources, and varying WOF of each
slow, cold extraction methods vs. hot extraction methods
several other ideas for sustainable mordant sources from non-mined materials
If you try this approach yourself, I’d love to hear about it. When referencing my research, please provide a link back to this page and/or credit me by name. Many thanks.
Cheers, Mel Sweetnam
Mamie’s Schoolhouse, Cape Breton Island, Nova Scotia, Canada (April 2020)
How To Cite This Study:
Sweetnam, M. (2020). Plant Derived Mordants. Mamie’s Schoolhouse. https://www.mamiesschoolhouse.com/blogarchive/2020/4/25/a-new-approach-to-plants-as-mordants
© 2020, Mel Sweetnam. All rights reserved.